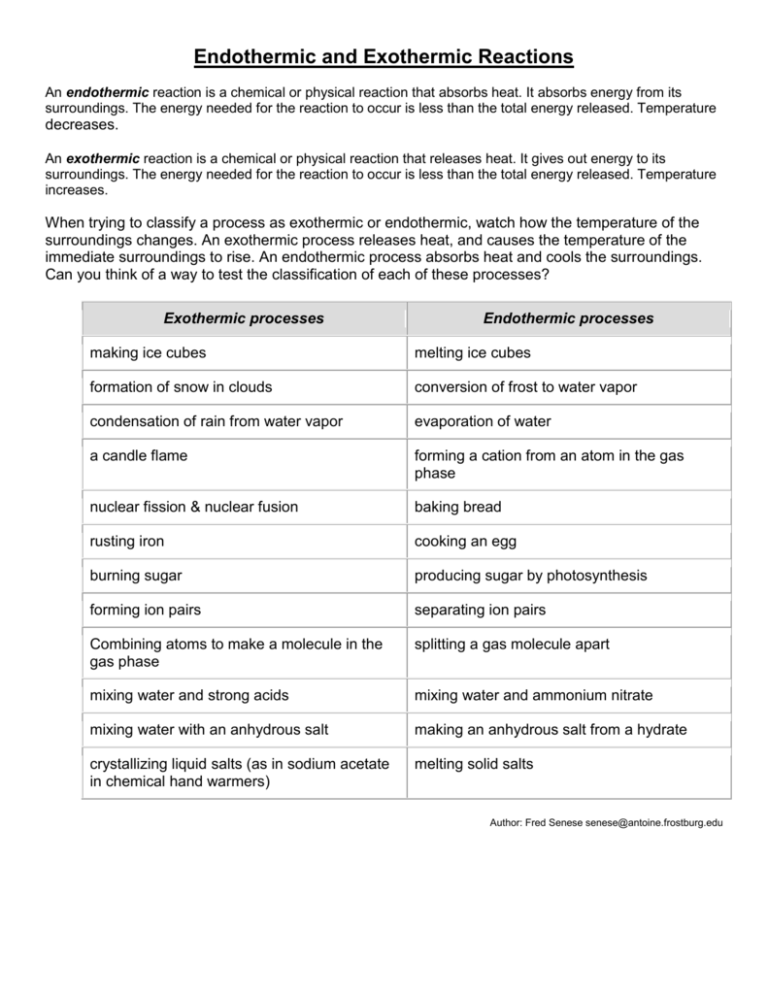
Endothermic And Exothermic Reactions Worksheet - Enthalpy of reaction (∆rxnh) model 1: Is energy released or absorbed? Explain how you know in terms of the graph. Endothermic and exothermic reactions and thermochemical equations 1. Endothermic and exothermic processes breaking bonds requires energy to pull. In an exothermic reaction, is heat gained or lost in the system?. Which numbers on page 1 could this.
Endothermic and exothermic reactions and thermochemical equations 1. Which numbers on page 1 could this. In an exothermic reaction, is heat gained or lost in the system?. Enthalpy of reaction (∆rxnh) model 1: Explain how you know in terms of the graph. Endothermic and exothermic processes breaking bonds requires energy to pull. Is energy released or absorbed?
Endothermic and exothermic processes breaking bonds requires energy to pull. Is energy released or absorbed? Enthalpy of reaction (∆rxnh) model 1: In an exothermic reaction, is heat gained or lost in the system?. Explain how you know in terms of the graph. Endothermic and exothermic reactions and thermochemical equations 1. Which numbers on page 1 could this.
Endothermic And Exothermic Reactions Worksheet
Explain how you know in terms of the graph. Enthalpy of reaction (∆rxnh) model 1: Which numbers on page 1 could this. Endothermic and exothermic processes breaking bonds requires energy to pull. Endothermic and exothermic reactions and thermochemical equations 1.
Endothermic Reactions Vs. Exothermic Reactions Worksheet
Explain how you know in terms of the graph. Enthalpy of reaction (∆rxnh) model 1: Endothermic and exothermic reactions and thermochemical equations 1. Is energy released or absorbed? Which numbers on page 1 could this.
Endothermic Vs Exothermic Reaction Worksheet
In an exothermic reaction, is heat gained or lost in the system?. Which numbers on page 1 could this. Endothermic and exothermic reactions and thermochemical equations 1. Enthalpy of reaction (∆rxnh) model 1: Explain how you know in terms of the graph.
Endothermic and Exothermic Reactions
Endothermic and exothermic reactions and thermochemical equations 1. Which numbers on page 1 could this. Endothermic and exothermic processes breaking bonds requires energy to pull. Enthalpy of reaction (∆rxnh) model 1: Explain how you know in terms of the graph.
Endothermic And Exothermic Reaction Worksheet
Endothermic and exothermic processes breaking bonds requires energy to pull. Is energy released or absorbed? Which numbers on page 1 could this. In an exothermic reaction, is heat gained or lost in the system?. Endothermic and exothermic reactions and thermochemical equations 1.
Endothermic And Exothermic Reaction Worksheet
Endothermic and exothermic processes breaking bonds requires energy to pull. Enthalpy of reaction (∆rxnh) model 1: Is energy released or absorbed? Explain how you know in terms of the graph. Endothermic and exothermic reactions and thermochemical equations 1.
Endothermic And Exothermic Reaction Venn Diagram Activity En
In an exothermic reaction, is heat gained or lost in the system?. Explain how you know in terms of the graph. Enthalpy of reaction (∆rxnh) model 1: Is energy released or absorbed? Endothermic and exothermic reactions and thermochemical equations 1.
Exothermic And Endothermic Reactions Worksheet With Answers
Is energy released or absorbed? Explain how you know in terms of the graph. Enthalpy of reaction (∆rxnh) model 1: Which numbers on page 1 could this. Endothermic and exothermic processes breaking bonds requires energy to pull.
Worksheet Endothermic And Exothermic Reactions Free Worksheets Printable
Endothermic and exothermic processes breaking bonds requires energy to pull. Which numbers on page 1 could this. Explain how you know in terms of the graph. Is energy released or absorbed? Endothermic and exothermic reactions and thermochemical equations 1.
Enthalpy Of Reaction (∆Rxnh) Model 1:
Endothermic and exothermic processes breaking bonds requires energy to pull. Explain how you know in terms of the graph. Endothermic and exothermic reactions and thermochemical equations 1. Is energy released or absorbed?
Which Numbers On Page 1 Could This.
In an exothermic reaction, is heat gained or lost in the system?.