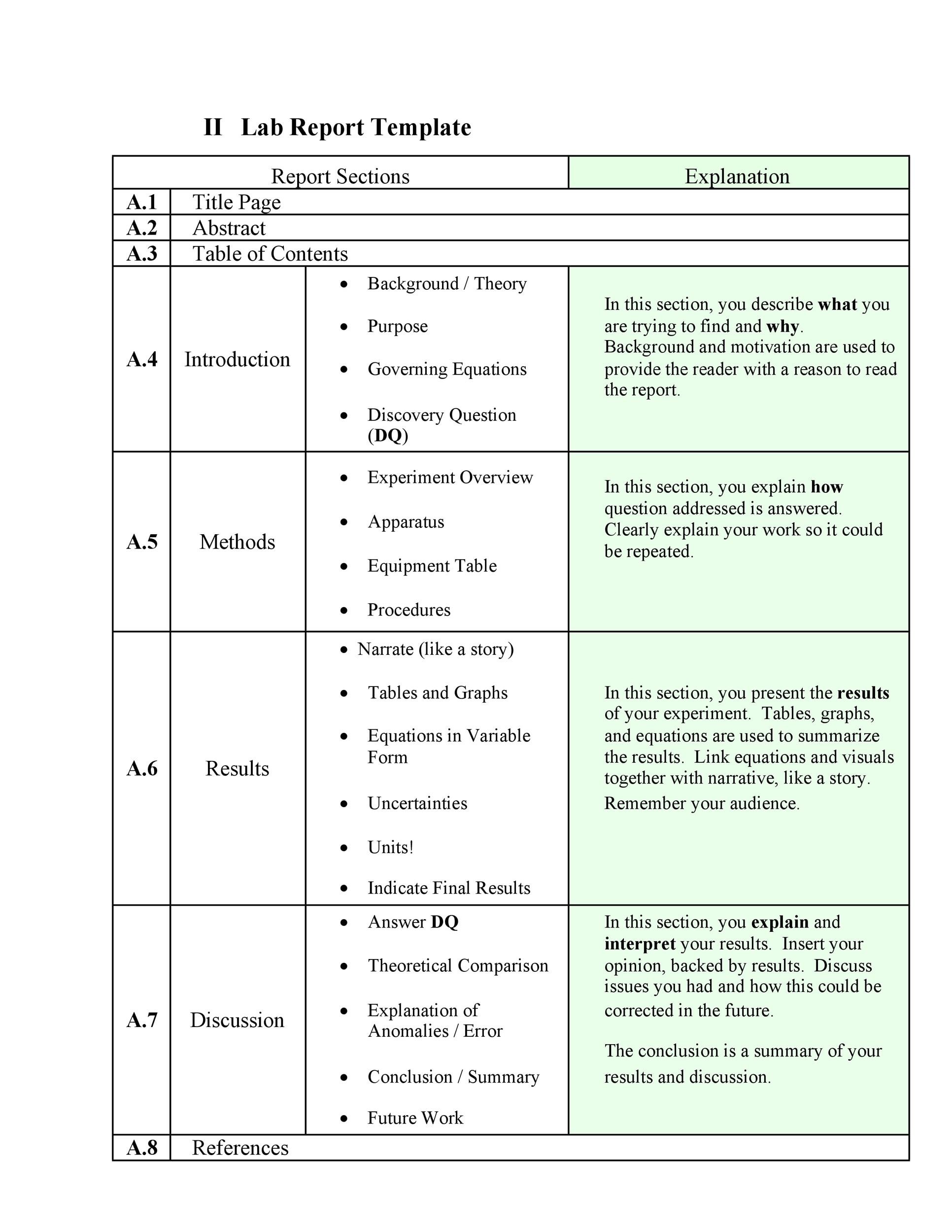
Lab Protocol Template - They follow the format of typical nih and industry multicenter protocols. The natural history/observational protocol template, the. Based on both fda regulation and oecd principles, this template can be used to do the following: Welcome to global health trials' tools and templates library. There are three templates to be used for observational research: (1) identify the test item, reference item, and test. The irb provides several protocol templates on this page. Please note that this page has been updated for 2015 following a quality check. Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or.
They follow the format of typical nih and industry multicenter protocols. The irb provides several protocol templates on this page. Please note that this page has been updated for 2015 following a quality check. Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. (1) identify the test item, reference item, and test. Welcome to global health trials' tools and templates library. Based on both fda regulation and oecd principles, this template can be used to do the following: The natural history/observational protocol template, the. There are three templates to be used for observational research:
Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. Based on both fda regulation and oecd principles, this template can be used to do the following: Please note that this page has been updated for 2015 following a quality check. There are three templates to be used for observational research: Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. (1) identify the test item, reference item, and test. The irb provides several protocol templates on this page. They follow the format of typical nih and industry multicenter protocols.
Biosafety Standard Operating Procedures Template
Please note that this page has been updated for 2015 following a quality check. (1) identify the test item, reference item, and test. There are three templates to be used for observational research: Based on both fda regulation and oecd principles, this template can be used to do the following: The irb provides several protocol templates on this page.
40 Lab Report Templates & Format Examples ᐅ TemplateLab
Please note that this page has been updated for 2015 following a quality check. Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. Based on both fda regulation and oecd principles, this template can be used to do the following: The irb provides several protocol templates on this page. There.
Lab Protocol Template
There are three templates to be used for observational research: Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. Based on both fda regulation and oecd principles, this template can be used to do the following: They follow the format of typical nih and industry multicenter.
Lab Protocol Template
Welcome to global health trials' tools and templates library. The natural history/observational protocol template, the. The irb provides several protocol templates on this page. Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. Please note that this page has been updated for 2015 following a quality check.
Laboratory Procedures Laboratories Experiment
Based on both fda regulation and oecd principles, this template can be used to do the following: (1) identify the test item, reference item, and test. The natural history/observational protocol template, the. Please note that this page has been updated for 2015 following a quality check. There are three templates to be used for observational research:
Protocol Template Biomedical
They follow the format of typical nih and industry multicenter protocols. There are three templates to be used for observational research: Based on both fda regulation and oecd principles, this template can be used to do the following: Welcome to global health trials' tools and templates library. Please note that this page has been updated for 2015 following a quality.
40 Lab Report Templates & Format Examples ᐅ TemplateLab
(1) identify the test item, reference item, and test. The natural history/observational protocol template, the. Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. Welcome to global health trials' tools and templates library. Based on both fda regulation and oecd principles, this template can be used to do the following:
Molecular biology Lab protocol
The irb provides several protocol templates on this page. There are three templates to be used for observational research: The natural history/observational protocol template, the. Based on both fda regulation and oecd principles, this template can be used to do the following: They follow the format of typical nih and industry multicenter protocols.
Free Lab Protocol Template Edit Online & Download
The natural history/observational protocol template, the. Please note that this page has been updated for 2015 following a quality check. Welcome to global health trials' tools and templates library. Based on both fda regulation and oecd principles, this template can be used to do the following: They follow the format of typical nih and industry multicenter protocols.
40 Lab Report Templates & Format Examples ᐅ TemplateLab
Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. The natural history/observational protocol template, the. There are three templates to be used for observational research: Based on both fda regulation and oecd principles, this template can be used to do the following: The irb provides several protocol templates on this.
There Are Three Templates To Be Used For Observational Research:
Describe whether results (study results or individual subject results, such as results of investigational diagnostic tests, genetic tests, or. They follow the format of typical nih and industry multicenter protocols. (1) identify the test item, reference item, and test. Based on both fda regulation and oecd principles, this template can be used to do the following:
Please Note That This Page Has Been Updated For 2015 Following A Quality Check.
The irb provides several protocol templates on this page. The natural history/observational protocol template, the. Welcome to global health trials' tools and templates library.