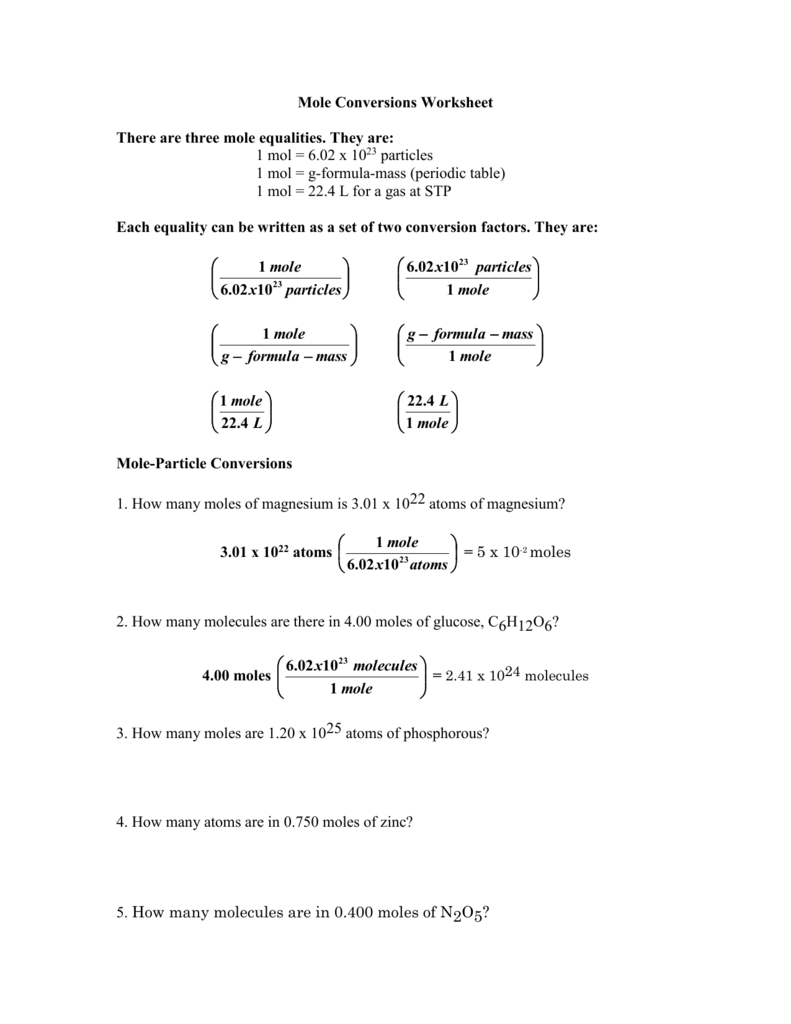
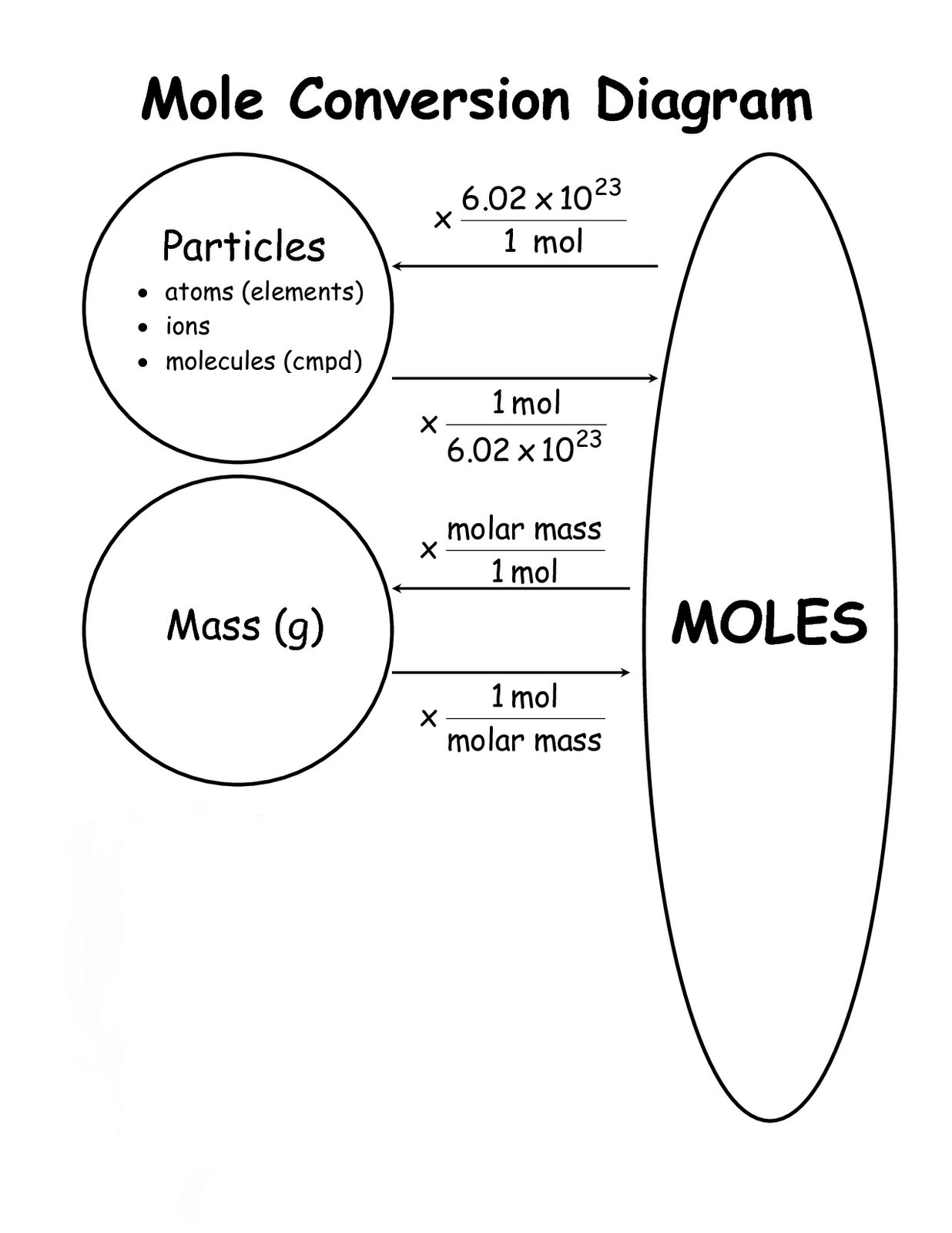
Mole Conversions Worksheet Working With Moles And Particles - We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Each equality can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. How many moles of magnesium is 3.01 x 1022 atoms of. If you can convert any of these things to. Find the number of molecules in 60.0.
How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of magnesium is 3.01 x 1022 atoms of. Find the number of molecules in 60.0. Each equality can be written as a set of two conversion factors. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. If you can convert any of these things to.
How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of magnesium is 3.01 x 1022 atoms of. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Each equality can be written as a set of two conversion factors. If you can convert any of these things to. Find the number of molecules in 60.0. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry.
Mole conversions worksheet working with moles and particles Message
If you can convert any of these things to. Find the number of molecules in 60.0. Each equality can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles.
Chemistry Mole Calculation Worksheet Interactive worksheet
Find the number of molecules in 60.0. How many moles of magnesium is 3.01 x 1022 atoms of. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Each equality can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of.
Mole To Mole Conversions Worksheet
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of magnesium is 3.01 x 1022 atoms of. We can convert from grams to.
Mole Conversions Worksheet Working With Moles And Particles
Find the number of molecules in 60.0. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Each equality can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of. 1 mole = molar mass (could be atomic mass from periodic table.
Mole To Mole Conversions Worksheet
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Find the number of molecules in 60.0. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of magnesium is 3.01 x 1022 atoms.
Mole To Particle Conversion Worksheets
How many moles of magnesium is 3.01 x 1022 atoms of. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. How many moles of magnesium is 3.01 x 1022 atoms of. Each equality can be written as a set of two conversion factors. Find the number of molecules in 60.0.
Free Printable Mole Conversion Worksheets
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. If you can convert any of these things to. How many moles of magnesium is 3.01 x 1022 atoms of. How many moles of magnesium is 3.01 x.
Moles to Particles Conversion Worksheet Atoms, Molecules, and
1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. How many moles of magnesium is 3.01 x 1022 atoms of. Each equality can be written as a set of two conversion factors. If you can convert any.
Mole Conversions Worksheet Working With Moles And Particles Printable
How many moles of magnesium is 3.01 x 1022 atoms of. Each equality can be written as a set of two conversion factors. How many moles of magnesium is 3.01 x 1022 atoms of. Find the number of molecules in 60.0. If you can convert any of these things to.
Mole Conversions Worksheet Working With Moles And Particles
How many moles of magnesium is 3.01 x 1022 atoms of. If you can convert any of these things to. Find the number of molecules in 60.0. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. Each.
Each Equality Can Be Written As A Set Of Two Conversion Factors.
How many moles of magnesium is 3.01 x 1022 atoms of. 1 mole = molar mass (could be atomic mass from periodic table or molecular mass) 1 mole = 22.4 l of a gas at stp ( you do not need to worry. We can convert from grams to moles, liters to moles (for gases), and atoms or molecules to moles. Find the number of molecules in 60.0.
If You Can Convert Any Of These Things To.
How many moles of magnesium is 3.01 x 1022 atoms of.